Ncert Class 12: Chemistry Chapter 1 The Solid State
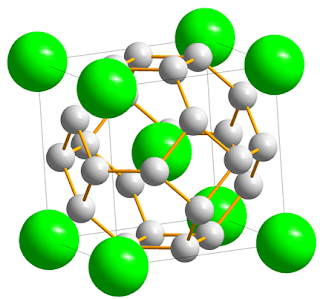

Solutions of Class 12 Chapter 1 Chemistry:-
1.1. Define the term 'amorphous'. Give a few examples of amorphous solids.
Solution:
Amorphous solids are those substances, wherein there is no regular arrangement of its constituent particles, (i.e., particles, atoms or atoms). The arrangement of the establishing particles has just short-range request, i.e., a regular and periodically repeating pattern is seen over brief distances just, e.g., glass, elastic, and plastics.
1.2. What makes glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Solution:
Glass is a supercooled fluid and an amorphous substance. Quartz is the crystalline type of silica (SiO2) in which tetrahedral units SiO4 are connected with each other in such a way that the oxygen atom of one tetrahedron is shared with another Si atom. Quartz can be converted into glass by softening it and cooling the dissolve rapidly. In the glass, SiO4 tetrahedra are participated in a random manner.
1.3 Classify each of the following solids as ionic, metallic, modular, network (covalent), or amorphous:
(i) Tetra phosphorus decoxide (P4O10) (ii) Ammonium phosphate, (NH4)3PO4 (iii) SiC (iv) I2 (v) P4 (vii) Graphite (viii), Brass (ix) Rb (x) LiBr (xi) Si
Solution:
Metallic : ( h ) Brass, ( i ) Rb
Molecular : (a) Tetra phosphorus decaoxide (P4O10), (d) I2, (e) P4.
Ionic : ( b ) Ammonium phosphate (NH4)3PO4, ( j ) LiBr
Amorphous : ( f ) Plastic
Covalent : ( c ) SiC, ( g ) Graphite, ( k ) Si
1.4 (i) What is meant by the term 'coordination number'?
(ii) What is the coordination number of atom
(a) in a cubic close-packed structure?
(b) in a body focused cubic structure?
Solution:
(i) The number of nearest neighbors of a particle are called its coordination number.
(ii) (a) 12 (b) 8
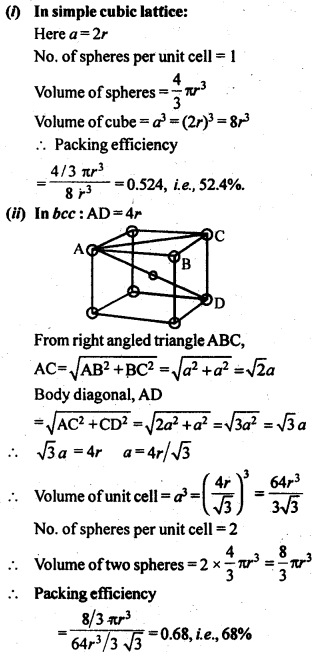
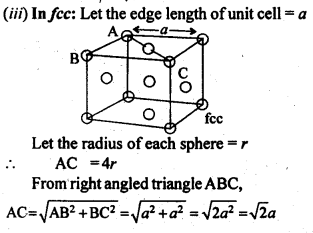
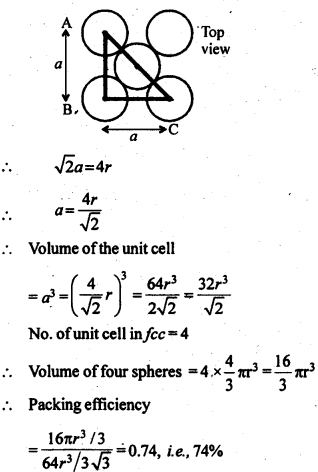
1.5. How can you determine the atomic mass of an unknown metal if you know its density and dimensions of its unit cell ? Explain your answer.
Solution:
We know the aspect and thickness of its unit cell.
Let,
The edge length of a unit cell = a
The volume of the cell = a3
Thickness = d
Nuclear mass = M
Mass of unit cell = No. of particles in unit cell x Mass of every molecule = Z × m
Mass of a particle present in the unit cell, m = M/Na
where Na is the Avogadro's number.
We know,
d = Mass of unit cell/Volume of unit cell = Zm/a3 = Z.M/a3Na
Consequently, Atomic mass, M =( da3 Na)/Z
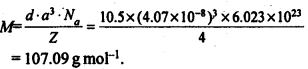
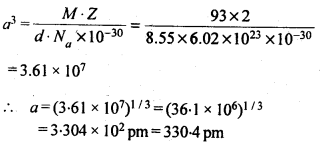

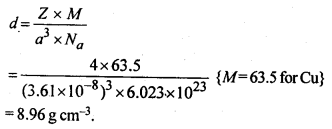
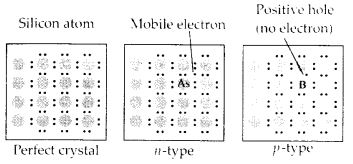
- Ge doped with In
- B doped with Si.
- Ge is group 14 element and In is group 13 element. Hence, an electron deficient hole is created and therefore, it is a p - type semiconductor.
- B is group 13 element and Si is group 14 element, there will be a free electron, So, it is a n-type semiconductor.
- between a conductor and an insulator
- between a conductor and a semiconductor?

- Schottky defect
- Frenkel defect
- Interstitial defect
- F-focuses.

- What is the length of the side of the unit cell?
- How many unit cells are there in 1.00 cm3 of aluminum?

Solution:
Let moles of NaCI = 100
∴ Moles of \(SrCl_{2}\) doped = \(10^{-3}\)
Each \(Sr^{2+}\) will replace two \(Na^{+}\) ions. To maintain electrical neutrality it occupies one position and thus creates one cation vacancy.
∴ Moles of cation vacancy in 100 moles NaCI = \(10^{-3}\)
Moles of cation vacancy in one mole
NaCI = \(10^{-3}\) × \(10^{-2}\) = \(10^{-5}\)
∴ Number of cation vacancies
= \(10^{-5}\) × 6.022 × \(10^{23}\) = 6.022 × \(10^{18} mol^{-1}\)
- Ferromagnetism
- Paramagnetism
- Ferrimagnetism
- Antiferromagnetism
- 12-16 and 13-15 group compounds.






.jpeg)


0 Comments
Thanks for comment.Please feedback us!